 I have seen the same weight loss results in many patients taking the HCG drops or HCG injections. I have had at least 50 patients do the drops for a week or two followed by the drops. There was no difference in hunger or weight loss. Many of my patients who have done both forms have reported less hunger and more energy on the HCG diet drops than while on the HCG hormone injections. A few patients have done very, very well on the HCG injections. I think its because those kind of patients are very serious, very careful and very dedicated.
I have seen the same weight loss results in many patients taking the HCG drops or HCG injections. I have had at least 50 patients do the drops for a week or two followed by the drops. There was no difference in hunger or weight loss. Many of my patients who have done both forms have reported less hunger and more energy on the HCG diet drops than while on the HCG hormone injections. A few patients have done very, very well on the HCG injections. I think its because those kind of patients are very serious, very careful and very dedicated.
Advantages of HCG Drops
Other reasons as to why I switched from HCG hormone injections to the HCG diet drops are:
- The HCG diet drops are easy to travel with; there is no need to carry around needles or alcohol pads.
- My HCG diet drops require no mixing. Many people do not know how to mix HCG and especially how to keep the preparation sterile.
- HCG drops are made with alcohol and bacteriostatic water. These solutions act as preservatives and keep the HCG effective.
- The HCG has an expiration date of at least 3 months if kept in the refrigerator.
- Some participants report more energy and less hunger on the HCG diet drops than with the HCG hormone injections.
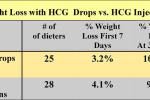
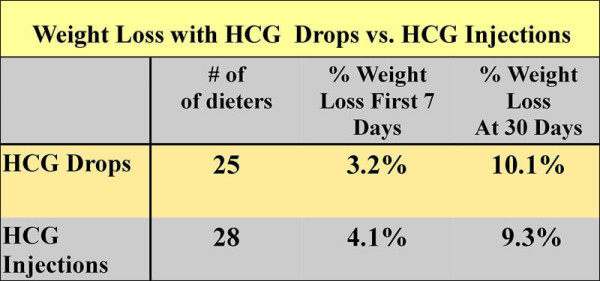
Amazing Weight Loss Results with HCG Drops
Look at some of the excellent results I have found with oral, sublingual HCG.
I recently reviewed more than 400 patients I have treated over the past year on HCG. Three quarters of the patients took the HCG orally, while one quarter decided to take injections. The all lost about the same. If you saw their starting weight, age, gender and the completion weight you could not pick out which ones did the shots or which ones the drops. One reason for almost equal results is due to the fact that that the patients who used drops took more than three times the dose that those that took injections. This was done to prevent potential problems with poor absorption orally. Also they took the drops two and often 2 times a day.
The average weight of the dieters in the little study reported was 225 lb in the Drop Group and 221 lb in the Injection group. Note that there was an initial faster weight loss with the injection group, but by the end of 30 days both groups lost about the same weight.
Read More on Oral HCG vs. Injectable HCG:
“Utility of an oral formulation of hCG for obesity treatment: A Double-Blind study”
Authors:
- Daniel O. Belluscio MD
- Leonor E. Ripamonte MD
Summary
To assess the validity of the hypothesis that hCG (Human Choriogonadotropin) mobilizes fat from deposits of fat, the authors designed a Double Blind Study performed on obese volunteers.
The result of this study suggests that oral administration of hCG, in the course of a Very-Low-Calorie Diet (VLCD), significantly decreases the total amount of subcutaneous body fat from specific deposits of fat. Since an increased amount of body fat is responsible for the genesis and maintenance of obesity, the authors suggest that hCG may be useful for obesity treatment.
Both Placebo and hCG-treated patients were managed with the same Very Low Calorie Diet. hCG-treated volunteers lost more body fat than their Placebo counterparts….
About forty years ago, a Physician published in the publication The Lancet, an unusual approach to the treatment of obesity (33,34). It consisted in the administration of small amounts of hCG (Human Choriogonadotropin), a substance secreted by the human placenta, to obese patients who were also put on a Very Low Calorie Diet (500 Kcal/day). He stated that obese individuals lost fat from fat deposits, felt better during treatment and improved the shape of their bodies.
The method was used, and misused, for about twenty years, until a series of tests (blind and open) led to the conclusion that this approach to obesity rendered the same results concerning weight loss both in Placebo and hCG-treated patients.(2,7, 14,15, 26, 32, 35,36,41).
Recent investigations demonstrate conclusively that it is possible to lose a great amount of weight at the expense of lean (instead of fat) tissue. Consequently, an important issue in any weight reduction program is knowing whether patients are losing lean body mass instead of fat.
Since no assessment of the fat to lean weight loss ratio was performed in those previous studies and, in our experience hCG has a metabolic action on fat masses, we have designed a new Research Protocol comprising parameters not included in previous studies.
Materials and methods for the study
All patients were Caucasian, ages ranging from 23 to 73 y.o (group P: 41 ± 13; group G1: 42 ± 12; group G2: 41 ± 14), a range of heights of 1,62 cm. to 1,81 cm., and overweight ranging from 25 to 49,9 on BMI Tables.
Since there were no published reports on the oral use of hCG, group G2 was administered twice the dose of G1, to assess if hCG concentration may affect obtained results.
The pharmacist prepared two types of vials: one containing saline solution (Na Cl 0,9% w/v), and the other containing a diluted and buffered solution of standardized hCG .
Vials were randomly labeled, each number corresponding to a patient. The pharmacist kept the codes in a sealed envelope. They were opened after completing the protocol.
To obtain a complete copy of the procedure please email your request at protocol@hcgobesity.org. You must be a Licensed Healthcare Professional to get a copy.
Test Parameters
Test parameters are as follows
A. Laboratory blood tests before (day 0) and after the study.
B. Subcutaneous weekly fat content assessments performed on different body areas (Skinfold thickness test), using a Lange (Cambridge,Mass) skinfold Caliper
- Tricipital region
- Anterior axillary line
- Subscapular
- Suprailiac
- Lateral thoracic region
- Supraumbilical region
- Infraumbilical region
- Inner thigh, 8 cm. below the pubic area.
- Areas above and below the patella.
C. Body Impedance, assessed with a Maltron (UK) portable device.
D. Subcutaneous fat on different body areas , assessed by a portable Ultrasound device (Keiko Ind., Japan).
Selected areas were:
- Tricipital region
- Inner thigh, 8 cm. below the pubic area.
- Areas above and below the patella.
E. Body circumference reduction in the course of the study. The following areas were measured with a metric tape measure:
- Wrist
- Chest
- Waist
- Abdominal region (at the level of the navel)
- Hips (maximum circumference)
- Thighs (8 cm. below the pubic line)
- Suprapatellar border
- Ankle
F. Irritability score during the treatment period, assessed through answers to a questionnaire sent to the volunteers weekly.
Assessments B,C,D and E were performed by the same observer throughout the study period, thus avoiding differences between individuals.
Study period
The study period lasted five weeks. At the end of the study, the envelope containing the codes for every patient were opened, and data collected were submitted to statistical variance and regression analysis.
Data analysis
For data analysis the following procedures were performed:
- Data were input into a database, filed in ASCII format.
- Analyses of frequency, mean, standard deviations, and standard errors.
- Variance, covariance and multiple regression analyses.
Study results
I. Skinfold thickness – Drug Concentrations
When comparing the obtained results before and after the study period, significant differences between Placebo and hCG treated volunteers were observed:
- For Dose 1:
1. Supraumbilical skinfold: F=2.24 p< 0.05 - For dose 2:
1. Supraumbilical skinfold: F=2.43 p<0.05
2. Infraumbilical fold: F= 8.99 p< 0.0001
3. Internal aspect of thighs: F=3.33 p< 0.01
4. Subscapular: F=4.22 p< 0.002
II. Skinfold thickness- Groups comparison
From the groups that received the active component, five volunteers did not complete the five weeks course of treatment, and three failed to attend at least one control session: Total eight patients.
Of the group that received the placebo, three patients did not complete the last two controls, and two failed to attend the last one. Total : 5 cases.
Analysis of data collected shows significant differences in the results of the following skinfold thickness tests between placebo and hCG- treated subjects:
- Subscapular region: p< 0.01
- Supraumbilical: p< 0.04
- Infraumbilical: p< 0.004
- Internal aspect of thighs: p<0.002
- Suprapatellar: p< 0.005
- Tricipital: p< 0.08
- Axillary anterior line: p<0.08
III. Ultrasound assessment
Ultrasound evaluations of subcutaneous adipose tissue from different groups revealed the following data:
- Inner thigh: p< 0.08
- Suprapatellar region: p< 0.08
IV.Weight loss, total fat and lean mass, impedanciomethry.
Impedance and body circumference measures revealed no statistically significant differences in weight loss, total fat and lean mass.
V. Mood behavior in the course of the treatment.
Study volunteers answered a questionnaire concerning their mood during the treatment period. The following statistical differences were observed between the responses received from hCG and Placebo-treated Groups:
- hCG-treated patients felt in a better the treatment: p < 0.03 at the 3rd. treatment week and p < 0.01 at the 5th.
- Sleep quality, regarding amount of hours and quality was improved : p < 0.06, at the 3rd. week of treatment.
- In a regular discussion with any member of the family they accepted more openly pt differences on opinions regarding the subject discussed: p< 0.01, at the 5th. treatment week.
- They were less irritable: p<0.001 at the 4th. treatment week.
- They were better able to cope when things did not go as they expected: p<0.05.
- They were less prone to quarrel for petty reasons: p<0.05.
- They were less prone to episodes of irritability : p< 0.005 at the 4th. week.
Discussion and conclusions
The hCG protocol for the treatment of obesity has been loved and hated for the last 40 years. In 1974, the FDA banned the use of hCG in the US based on a series of studies maintaining that weight loss was similar both in the Placebo and hCG-treated groups. Our study corroborates those previous findings.(2,7, 14,15, 26, 32, 35,36,41).
However, all the the reports on the subject of hCG and obesity are not negative: studies from Asher WL, Harper HW, Bradley P., Bradley P., Gusman HA, Komarnicka R, et al., Vallini A, et al, Veilleux H, et al., suggest that hCG exerts a lipid-mobilizing action.
Unfortunately, previous studies based their conclusions on the fact that weight loss was similar both in hCG and Placebo treated patients. But weight on a scale is only one of the tools suitable to describe obesity: A more accurate description recently coined is that obesity is the accumulation of body fat above predefined standards.
If obesity can be defined as an excess of body fat, then it follows that any pharmacological intervention on the disease should assess whether the drug in question can mobilize fat from fat deposits.
According to our preliminary study, hCG oral administration decreases the amount of body fat from certain body regions to a statistically significant level. This is the first study published on the oral use of hCG for the treatment of obesity.
This hCG metabolic action has been previously suggested by the studies of Fleigelman R, Fried GH., Scaffidi V., Veilleux H, et al., Yanagihara Y. , Yanagihara Y., Yanagihara Y..
Therefore, it seems that hCG can accelerate fat mobilization from certain fat deposits in the course of a weight reduction program including a Very Low Calorie Diet (VLCD).
Subcutaneous adipose tissue reduction could be accomplished by lipogenesis inhibition at the level of the fat cell membrane (see : adipose tissue metabolism). Since administered hCG accumulates at the hypothalamic region, we speculated that hCG metabolic actions could be exerted through the release of a lipolytic substance located in that area.
What is the significance of this finding within the context of obesity?
Adipose tissue reduction should be the goal in any weight reduction program, rather than appetite or anxiety reduction. This study suggests that the oral use of hCG helps to mobilize fat from certain fat deposits, but poses several as yet unsolved questions:
- Why was circumference reduction similar in both groups while the hCG treated group significantly decreased the amount of subcutaneous body fat? Our hypothesis is that they gained total body water and/or lean mass.
- Why did hCG treated volunteers not lose more weight despite a significant reduction in subcutaneous fat?. We have no explanation for these data.
In our opinion, further studies should be performed to ascertain the unresolved issues in this preliminary report.
We followed patients for a 8 mo. following treatment. A preliminary conclusion can be drawn: At 8 mo. post-treatment, more of the hCG-treated patients maintained their weight reduction that their placebo-treated counterparts (p< 0.003).
No side effects were reported with the oral use of hCG.
We suspect that only a fraction of hCG is absorbed, and this fraction might be responsible for the metabolic activity we have observed in our patients.
Since hCG contains IR beta-endorphin (17), we hypothesize that the improvement in our volunteers’ daily mood might be related to ß-endorphinergic activity at hypothalamic level. When hCG-treated volunteers where compared to the placebo-treated group regarding their mood during the treatment, the former were less irritable, felt in a better mood throughout the treatment, and their irritability responses were significantly decreased.
According to previous studies, subcutaneous adipose tissue reduction could be accomplished by an inhibition of lipogenesis at the level of the adipocyte membrane (see : adipose tissue metabolism). Since administered hCG accumulates at the hypothalamic region, we speculated that hCG metabolic actions could be exerted through the release of a lipolytic substance located in that area. hCG has no direct action upon fat cells.”


Leave a Comment